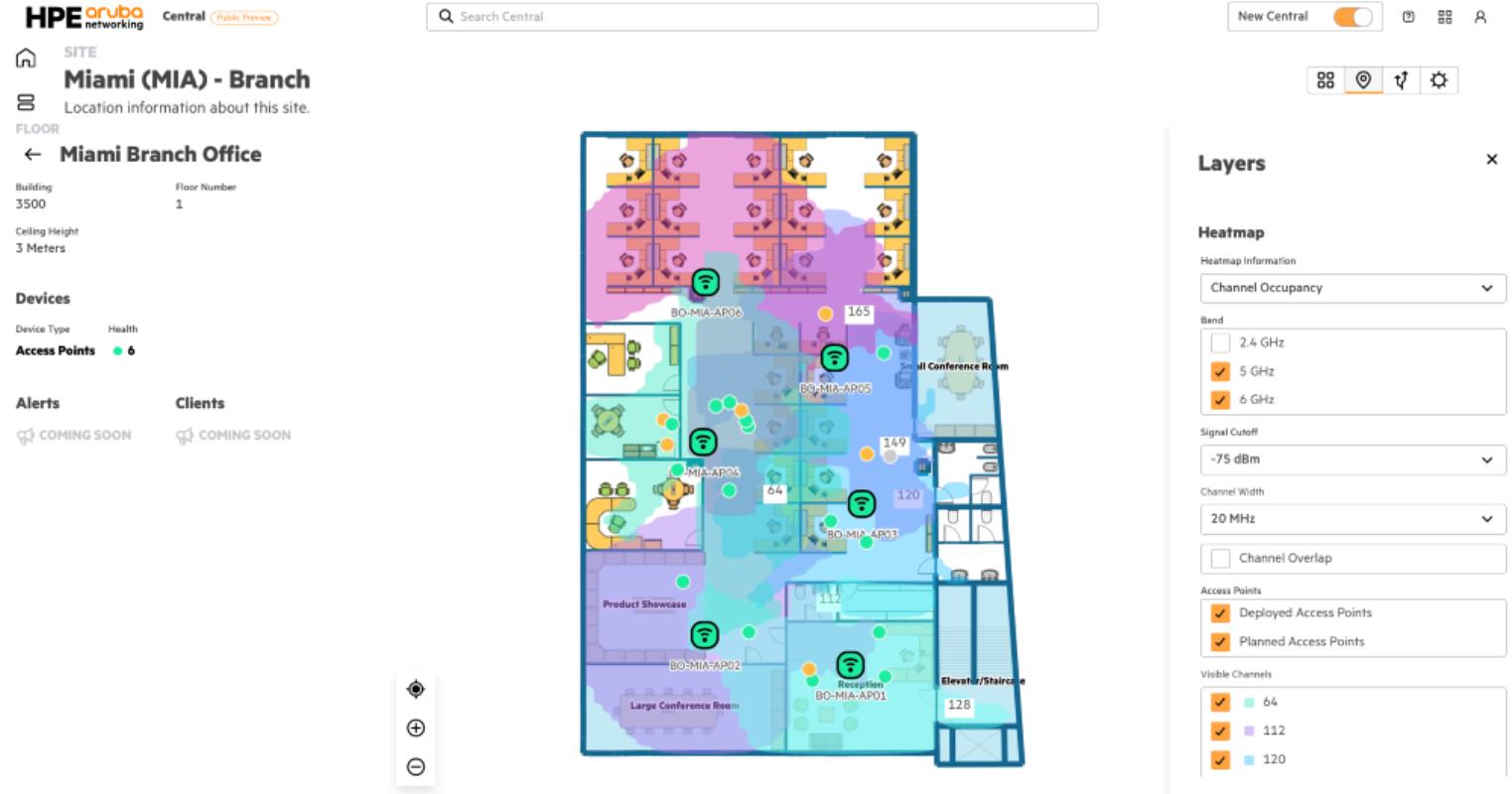
The National Health Regulatory Authority (NHRA) has approved Regn-Cov2 for emergency use to treat Covid-19 cases with mild to moderate symptoms.
Regn-Cov2 developed by Regeneron and Roche, contains a combination of Casirivimab and Imdevimab (monoclonal antibodies) and is projected to block viral attachment and entry into human cells.
It also received emergency use approval from the US’ FDA to treat Covid-19 in non-hospitalised adults and adolescents aged 12 years and older, who weigh at least 40kg, and those in the high-risk category.
The drug also received a positive review from the European Medicines Agency after their analysis of its quality, safety and efficacy.
Read More - www.gdnonline.com