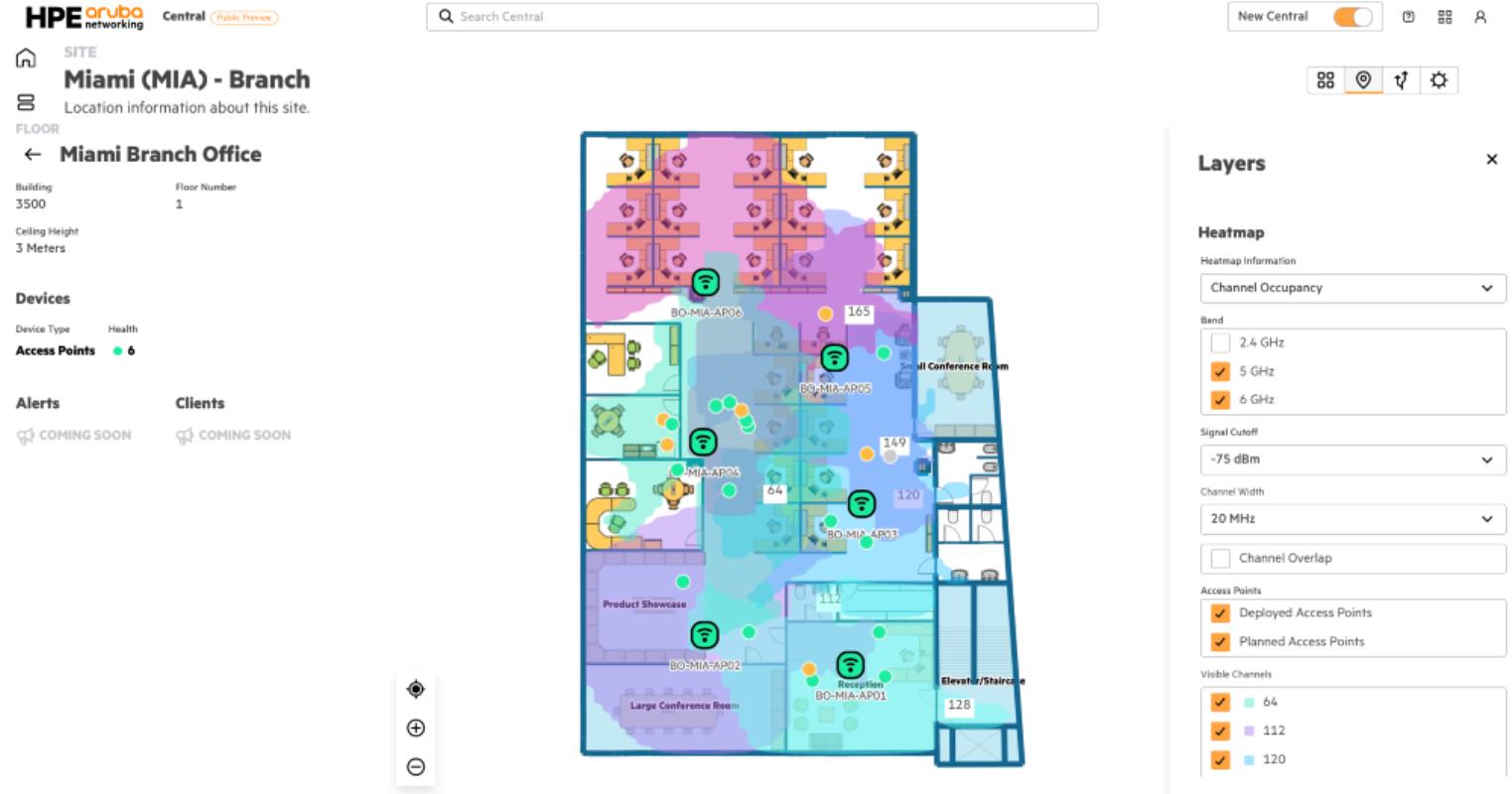
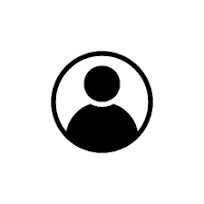
The National Health Regulatory Authority (NHRA) today approved the emergency use of the oral Covid-19 antiviral, Paxlovid.
The medication has been authorised for individuals 18 years old and above who suffer from mild to moderate symptoms and are at an increased risk of developing severe Covid-19 that may lead to mortality.
Click here to read more.